The human brain is biologically the most intricately protected organ and, paradoxically, remains the most challenging 'impregnable fortress' for treatment due to its protective mechanisms. Among them, glioblastoma (GBM) is classified as the most lethal and destructive malignant tumor in the field of neurosurgery. On February 2, 2026, Professor Ahn's team from the Department of Neurosurgery at Catholic University Seoul St. Mary's Hospital embarked on a bold challenge to solve this historical dilemma in glioblastoma treatment by initiating research on 'Intranasal Administration-Based Adoptive Immune Cell Therapy', selected for the Ministry of Science and ICT's 'New Research - Pioneering Research' project.
Glioblastoma is the most common primary malignant brain tumor occurring in adults, accounting for about 15% of all brain tumors and approximately 45-50% of malignant brain tumors. In South Korea, it occurs at a rate of about 5 people per 100,000 annually, with 600 to 800 new patients diagnosed each year.
Extremely Low Survival Rate: Even with the active implementation of the current standard treatment, 'Post-Surgical Temozolomide Chemoradiotherapy (Stupp Protocol)', the average survival time (Median Overall Survival) is only 12 to 15 months. The 5-year survival rate is less than 7 to 10%, indicating that it is one of the worst cancers that modern medicine has yet to conquer, alongside pancreatic cancer.
High Recurrence Rate: Glioblastoma exhibits an invasive growth pattern, so even if all visible tumors are removed surgically, microscopic cancer cells that have infiltrated surrounding normal brain tissue remain, leading to over 90% of patients experiencing recurrence. After recurrence, there are no appropriate standard treatment options, and survival time is shortened to a matter of months.
The reasons for the repeated failures in glioblastoma treatment are primarily due to two enormous barriers.
Physical Barrier (Blood-Brain Barrier, BBB): The endothelial cells of the brain's blood vessels are connected by tight junctions, blocking over 98% of substances in the blood. Most drugs with a molecular weight of over 400 daltons (Da) cannot pass through this barrier, and particularly, large molecules such as antibody therapies or immune cells have a brain parenchyma delivery rate of less than 0.1% when administered systemically (intravenously). This creates a 'delivery failure' issue, where even powerful anticancer drugs cannot be delivered into the brain.
Immunological Barrier: Glioblastoma is a representative 'cold tumor'. There is little infiltration of T cells within the tumor, and tumor cells secrete strong immunosuppressive substances (such as TGF-β) that neutralize even the infiltrating immune cells. As a result, immune checkpoint inhibitors (such as PD-1 inhibitors) that have shown miraculous effects in melanoma and lung cancer have experienced devastating failures in glioblastoma monotherapy.
Professor Ahn's team's research proposes an innovative approach that simultaneously bypasses and targets these two barriers, namely the innovative approach of injecting 'enhanced immune cells' through a bypass route called 'Nose'.
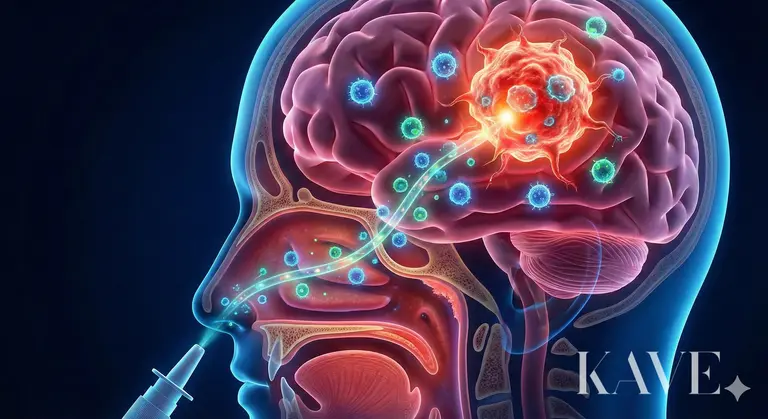
Humanity's efforts to overcome the blood-brain barrier (BBB) have been ongoing for decades. The method of pouring in high doses of anticancer drugs has caused systemic toxicity (bone marrow suppression, liver toxicity), and the approach of temporarily opening the BBB using mannitol carries the risk of brain edema. Recently, techniques using ultrasound to locally open the BBB have been attempted, but they still require equipment and involve complex procedures. The intranasal delivery that Professor Ahn's team focuses on utilizes the only anatomical pathway in the human body that directly connects the external environment to the central nervous system (CNS).
The olfactory epithelium in the upper nasal cavity has exposed olfactory nerve cells. The axons of these nerve cells connect directly to the olfactory bulb of the brain through tiny holes in the cribriform plate.
Perineural Space: Drugs or cells can move into the neurons (Intraneuronal), but they are more likely to move through the perineural space that surrounds the nerve bundles. This space is continuous with the subarachnoid space where cerebrospinal fluid (CSF) flows, allowing passage into the CSF without crossing the blood-brain barrier.
Speed: Transport within the nerve is a slow process that takes days, while extracellular transport through the perineural space acts as a highway that can reach brain tissue within minutes.
If the olfactory nerve connects to the front of the brain (near the frontal lobe), the trigeminal nerve, which is widely distributed throughout the nasal mucosa, connects to the brainstem and pons, the central part of the brain. Professor Ahn's research aims to utilize both of these pathways to deliver immune cells not only to the anterior part of the brain but also to tumors located deep within. The research team had already verified the potential through animal experiments prior to the selection of this national project. In previous studies, when immune cell therapies such as CAR-T (Chimeric Antigen Receptor T cells) were administered intranasally, it was confirmed that these cells effectively migrated to the brain tumor site and exhibited significant anti-tumor effects. This was possible because living cells utilized their 'homing' ability to actively seek out the tumor, following chemokine signals, rather than simply diffusing like drugs.
'Adoptive Cell Therapy (ACT)' is a treatment method that extracts immune cells from the patient's body, enhances/modifies them, and then reinjects them. Professor Ahn's team uses highly engineered cells tailored to the characteristics of glioblastoma, rather than simple immune cells. Recently, CAR-T cells, which have shown miraculous effects in hematological cancers, are T cells equipped with receptors (CAR) that recognize specific proteins on the surface of cancer cells.
Targets: In the case of glioblastoma, EGFRvIII (a mutated protein that is absent in normal cells but present in glioblastoma) and IL13Rα2 are the main targets.
Advantages of Intranasal Delivery: CAR-T cells administered intravenously face the issue of getting trapped in the lungs or liver (First-pass effect), but with intranasal delivery, they can go directly to the brain without systemic loss, allowing for high therapeutic effects even with lower doses.
Professor Ahn has also focused on research involving NK cells (Natural Killer cells) and gamma-delta T cells. Glioblastoma can hide its target proteins (Antigen Loss) to evade attacks from CAR-T cells, but NK cells are innate immune cells that can attack cancer cells while ignoring these evasion mechanisms. The research team is building a platform that can carry various 'weapons' such as CAR-T, NK, and gamma-delta T cells through the intranasal route, depending on the patient's characteristics.
One of Professor Ahn's team's most original achievements is the treatment method using 'genetically modified stem cells'. According to research published in the international journal Biomedicine & Pharmacotherapy in 2025, the team equipped mesenchymal stem cells (MSCs), which have excellent tumor-tropism, with the interleukin-12 (IL-12) gene.
Mechanism: MSCs administered intranasally or locally penetrate deeply into the tumor.
Action: MSCs secrete IL-12 within the tumor. IL-12 is a powerful immune-activating cytokine that awakens surrounding dormant NK cells and T cells to attack the tumor.
Results: When this treatment was combined with PD-1 immune checkpoint inhibitors, a 50% complete remission rate was observed in mouse models, and even after reintroducing cancer cells post-treatment, the 'immunological memory' effect was proven, showing no recurrence.
Professor Ahn's team's research is not an isolated attempt but is at the forefront of the global competition for next-generation brain tumor treatment technologies. Compared to major research teams in the United States, the approach of the Seoul St. Mary's Hospital team holds a unique position in combining 'non-invasive' and 'cell engineering'.
In 2025, researchers from the University of Pennsylvania announced groundbreaking clinical results in Nature Medicine. They successfully reduced tumor size by administering 'dual-target CAR-T' that simultaneously targets EGFRvIII and IL13Rα2 to patients with recurrent glioblastoma.
Limitations: The Penn team drilled holes in the skull to send cells to the brain and inserted a tube called an 'Ommaya reservoir' for direct injection into the ventricles. While this is a definitive delivery method, it requires surgery, poses infection risks, and causes significant pain to patients.
Comparison: Professor Ahn's team's intranasal delivery method has the potential to be a 'Game Changer' that can achieve similar effects without such surgical procedures.
The research team at the University of Washington announced in 2025 in PNAS the results of treating glioblastoma by administering nanoparticles called 'Spherical Nucleic Acids' intranasally.
Approach: Immune-activating substances were coated onto gold nanoparticles and administered intranasally to activate the immune environment of the brain tumor.
Comparison: While WashU's method focuses on the delivery of 'drugs (nanoparticles)', Professor Ahn's team delivers 'cells'. Unlike drugs, cells can move, proliferate, and respond to changes in the tumor, making them potentially more advantageous in overcoming the complex glioblastoma microenvironment.
This research, supported by the Ministry of Science and ICT (300 million won over three years), focuses on securing concrete data for clinical application beyond basic experiments.
Pathway Mapping: Using fluorescently labeled immune cells, it visually identifies which pathway the cells primarily use between the olfactory nerve and trigeminal nerve during intranasal delivery and how much accumulates in which part of the brain.
Cell Engineering: It incorporates technology to overexpress proteins (e.g., chemokine receptor CXCR4) that help immune cells adhere well to the nerve mucosa or move more quickly through the perineural space. This is to prevent cells from being expelled due to runny noses or sneezing and to maximize the efficiency of movement to the brain.
Safety and Toxicity Assessment: It verifies whether the immune cells that reach the brain cause neurotoxicity by attacking normal brain cells or trigger excessive inflammatory responses.
In an interview, Professor Ahn stated, "Once this research is established, it could develop into a 'universal platform' applicable not only to glioblastoma but also to other central nervous system diseases such as brain metastasis, Alzheimer's, and Parkinson's disease." This technology of sending 'cells' non-surgically to the brain, rather than drugs, can also be applied to deliver stem cells to regenerate nerves in degenerative brain diseases.
Glioblastoma has been the graveyard of countless new drugs over the past few decades. The impregnable fortress of the blood-brain barrier and the immunological characteristics of cold tumors have neutralized existing anticancer strategies. However, Professor Ahn's team's 'Intranasal Administration-Based Adoptive Immune Cell Therapy' is being evaluated as an innovative breakthrough to overcome this deadlock.
If this research is successfully carried out, the following future will open up.
Improvement in Patients' Quality of Life: Without repetitive craniotomies or hospitalizations, patients will be able to receive immune cell therapy in outpatient settings through nasal sprays or drops, significantly alleviating their suffering.
Increased Treatment Efficiency: By directly targeting high concentrations of immune cells to the brain tumor site without systemic side effects that occur with intravenous administration, treatment effects can be maximized.
Prevention of Recurrence: The possibility of fundamentally blocking recurrence, the biggest problem of glioblastoma, opens up through the formation of immune memory.
Professor Ahn's research is not merely about developing new drugs but is a convergent approach that innovates the 'pathway' for drug delivery and designs 'cells' optimized for that pathway. The three-year research period starting in 2026 will be a crucial turning point for Korea to leap forward as a 'First Mover' in the global neuro-oncology field. We are now witnessing a historical inflection point where glioblastoma, once deemed incurable, is transforming into a 'manageable chronic disease' or 'curable disease'.